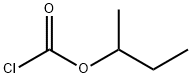
English name | sec-Butyl chloroformate仲丁基氯甲酸酯 |
Synonyms | SBCF;SEC-BUTYLCHLOROFORMATE;carbonochloridicacid,1-methylpropylester;s-ButylChloroformate;Butan-2-ylcarChemicalbookbonochloridate;Chloroformicacidsec-butyleste;sec-Butylchlorocarbonate;Chloridocarbonicacidsec-butylester |
CAS | 17462-58-7 |
EINECS | 241-475-6 |
Molecular formula | C5H9ClO2 |
Molecular weight | 136.58 |
Density | 1.081±0.06 g/cm3(Predicted) |
Boiling point | 126 °C |
HS | 2915900090 |
Package | (According to user requirements) |
Dangerous goods transport No. | UN 2742 |
Hazard Class | 6.1(a) |
Packing Group | II |
USE | Sec-butyl chloroformate is an organic chemical raw material used in the synthesis of certain useful compounds. 1. Application of sec-butyl chloroformate in the synthesis of the insecticide hydroxypiperate includes the following steps: 1) Preparation of 2-ethanolpiperidine: 2-glycolpyridine was dissolved in polar solvent with the amount of 1 ~ 5 times of its weight. Under the action of high efficiency hydrogenation catalyst with the amount of 2-glycolpyridine with the weight of 0.1 ~ 10%, the reaction was completed when the content of 2-glycolpyridine was less than 0.5% of the total amount under the pressure of 1 ~ 10Mpa hydrogen and the temperature of 50 ~ 120℃ for 6 ~ 12 hours. After the catalyst was removed by filtration, the solvent was recovered under pressure, and the remaining solvent was 2-ethanolpiperidine. 2) Preparation of hydroxypiperate crude product: 2-ethanolpiperidine was dissolved in a non-polar solvent that was 1-5 times its weight, and the reaction temperature was controlled at 40 ~ 80℃. At the same time, sec-butyl chloroformate and 1-20% alkali solution were added, and the molar ratio was base: Sec-butyl chloroformate ∶ 2-ethanolpiperidine = 1-1.5 ∶ 1-1.3 ∶1, and the drip time was controlled within 1 ~ 6 hours. The water layer was separated. The organic phase was washed two times with dilute acid with a concentration of 1-10% and an dosage of 1-3 times the weight of 2-ethanolpiperidine. After two times of water washing, the solvent was recovered under pressure. 3) Refining and purification of hydroxypiperate: the amount of antioxidants of 1-3% by weight is added to the crude product of hydroxypiperate, and the product of hydroxypiperate with more than 98% content is obtained by distillation under the high vacuum of less than 5mmHg and at the high temperature of 140 ~ 150℃. 2. Sec-butyl chloroformate is a kind of insect repellent, the preparation method includes the following steps: 1) the preparation of 2-hydroxyethylpyridine: 2-methylpyridine, paraformaldehyde and catalyst are added to the reaction bottle, heated and stirred reaction, vacuum distillation, collection of 130 ~ 150℃/10 ~ 20mmHg fraction, 2-hydroxyethylpyridine product; The mass ratio of -methylpyridine to polychemicalbook formaldehyde was 1∶(0.2 ~ 0.4); The amount of catalyst added according to the mass percentage of 2-methylpyridine is 2% ~ 5%; The reaction temperature is 60 ~ 100℃. The stirring reaction time was 15-20h. The catalyst is sodium carbonate or potassium carbonate. 2) Preparation of 2-hydroxyethylpyridine: dissolve 2-hydroxyethylpyridine in solvent, add catalyst, and heat reaction with hydrogen gas. Gas chromatography analysis shows that the reaction stops when the content of 2-hydroxyethylpyridine is less than 1% of the total amount. The catalyst is removed by filtration of the reaction liquid, and the solvent is recovered by distillation. The mass ratio of 2-hydroxyethylpyridine to solvent is 1∶(2 ~ 5); The addition amount of catalyst is 1%-5% of 2-hydroxyethylpyridine by mass percentage. The hydrogen pressure is 2 ~ 4Mpa; The reaction temperature is 60 ~ 80℃. The reaction time was 4 ~ 8h. The solvent is one of toluene, ethyl acetate, tetrahydrofuran or ethanol; The catalyst was Raney nickel (SC-4100) or 10% palladium carbon. 3) Preparation of Ecalidin: The 2-hydroxyethylpiperidine was placed in the reaction bottle, and the alkali solution with a concentration of 20% was added to stir the mixture, and sec-butyl chloroformate was added to the mixture. After addition, the mixture was continued to stir for two hours until the end of the reaction. The water layer was separated, and the organic phase was washed with 10% acid solution three times, and then washed twice. The product is eckaridin. The molar ratio of 2-hydroxyethylpiperidine to base and s-butyl chloroformate was 1∶(1.5 ~ 2.0)∶(0.95 ~ 1.0). The time of drip adding sec-butyl chloroformate is 60 ~ 100min, and the temperature of continuous stirring reaction is 30 ~ 50℃. The base is one of sodium carbonate, sodium hydroxide, or sodium bicarbonate. The acid solution is one of hydrochloric acid solution, sulfuric acid solution or acetic acid solution, and the dosage is 3 times the mass of 2-hydroxyethylpiperidine. |






