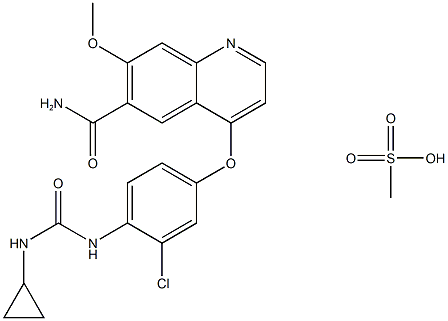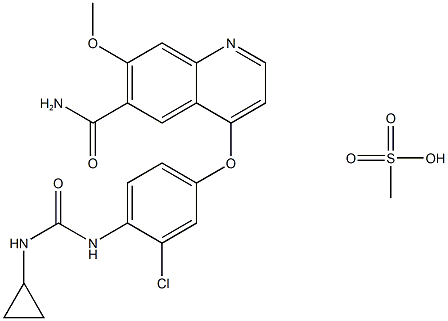
English name | Lenvatinib mesylate 甲磺酸乐伐替尼 |
Synonyms | lenvatinibMethanesulfonate;E7080Mesylate;4-[3-Chloro-4-[[(cyclopropylamino)carbonyl]amino]phenoxy]-7-methoxy-6-quinolinecarboxamidemonomethanesulfonate;4-[3chloro-4-(N'-cycloChemicalbookpropylureido)phenoxy]-7-methoxyquinoline-6-carboxamidemethanesulfonate;CAT#A863437;LenvatinibMesylate,AmadisChemicalofferCAS#857890-39-2;lenvatinibMesylate;E7080;E-7080;E7080 |
CAS | 857890-39-2 |
EINECS | 812-398-0 |
Molecular formula | C22H23ClN4O7S |
Molecular weight | 522.95862 |
Chemical formula |
|
Melting point | >220°C (dec.) |
Solubility | DMSO (Slightly), Methanol (Slightly) |
Storage condition | -20°C Freezer |
Appearance | Solid |
Colour | White to Off-White |
Use | It is a multi-target tyrosine kinase inhibitor, which may play a role in a variety of cancers. Therefore, Eisai is still conducting research on the use of Lovatinib in other cancers, such as hepatocellular carcinoma, non-small cell lung cancer, melanoma, breast cancer, lymphoma, ovarian cancer, etc. After drug transfer data retrieval, there are 62 clinical studies related to Lovatinib Chemicalbook. In July 2017, Eisai submitted an application to the US FDA and the European Medicines Agency for the marketing of Levaritinib as a first-line treatment for advanced hepatocellular carcinoma. In clinical trials, Levaratinib was shown to be superior to Sorafenib, the current standard treatment for advanced hepatocellular carcinoma. |
Upstream raw materials | Levaritinib methyl sulfonic acid |